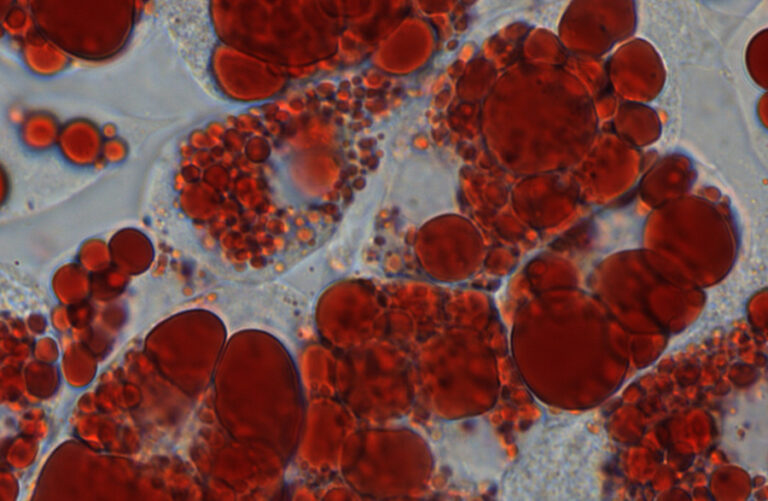
New research led by Roger Cox shows how overexpression of the Fto gene makes mice fat, which could explain why there is an association between an FTO variant and obesity in people.
Role of Fto gene in obesity revealed New research led by Roger Cox shows how overexpression of the Fto gene makes mice fat, which could explain why there is an association between an FTO variant and obesity in people. Fat cells (stained red) produced abundantly in mice where the Fto gene is highly activated While the role of a healthy diet and plenty of exercise in preventing obesity is well known, the influence of our genetic makeup is less clear. We all know some people who struggle to keep their weight under control and others who eat to excess, yet never seem to gain any weight. The answer to why there is such a difference could lie in the role of genes such as FTO. The fat mass and obesity-associated (FTO) gene was the first gene shown to play a role in human obesity on a large scale. Initially, an association was found between obesity and a specific variant of the FTO gene in humans, but this did not show cause and effect. However, it was followed by studies in mice that showed an overexpressed Fto gene resulted in excessively fat mice, particularly when they were fed a high fat diet. Conversely, mice lacking a functional Fto gene were found to be especially lean. However, the mechanism by which Fto acted remained elusive, and many questions remained regarding the role it played in regulating fatness and weight gain. The process of generating new fat cells, or adipocytes, is referred to as adipogenesis. Roger Cox’s team at MRC Harwell, together with researchers at the University of Oxford, have now shown that Fto acts upon key processes that occur during the early stages of adipogenesis, causing more fat cells to be produced. The work, jointly funded by the Medical Research Council and Wellcome Trust, is published in Nature Communications. Understanding the obesity ‘epidemic’ In the west we are currently in what many are calling an obesity ‘epidemic’ (although not infectious), with more people being classed obese than ever before. To figure out why this is happening, we need to understand every aspect of it, including how our genes could make some of us more prone to getting fat. While so far in people we only know there is an association between a variant of FTO and being obese, how the variant predisposes to obesity is poorly understood. Many early studies on FTO function focussed on food intake, but this study adds to a growing number of studies pointing to a functional role in fat itself. This study suggests Fto has a major influence on our propensity to gain weight, by influencing the amount of fat cells we produce in response to overeating, and could help to explain why people are becoming obese. “It has emerged that a number of genes in the FTO locus may co-ordinately be involved in determining obesity,” said Samantha Laber, a DPhil student joint first author on the study, “but our work and that of others clearly shows a central role for FTO in adipogenesis.” Elucidating the role of Fto The Fto gene product is a nucleic acid demethylase, an enzyme which removes methyl groups from DNA and RNA. It is known to regulate splicing of mRNA from many genes by affecting the ability of the splicing factor to bind to it. One of these genes is RUNX1T1, which as others have shown can be spliced differently to give either a long or short form. The short form promotes adipogenesis. The researchers therefore sought to determine whether Fto acts via RUNX1T1 to increase fat production in mice. The proposed role of FTO – boosting an early stage of fat cell production via the short form of RUNX1T1 Mice with Fto overexpressed (causing an abundance of the FTO enzyme) gain weight easily, but only become noticeably bigger when they are older, as it takes time for the fat to accumulate. However, the researchers found that the fat of younger mice looked different under the microscope, with lots of little fat cells, an indication that an early stage of adipogenesis was already taking place. To study how Fto activity affects adipogenesis, the researchers took mouse embryonic fibroblasts and early ‘preadipocytes’ from mice with Fto overexpressed and knockout mice (which lack Fto). These were cultured in a special cocktail that caused them to develop into mature adipocytes. Genes with a key role in adipogenesis were less active than normal in the cells from the knockout mice, but were overly active in the cells from the mice with overexpressed Fto. As they saw in the fat tissue from the mice, the cells cultured from the mice with overexpressed Fto produced lots of little adipocytes, a sign of an early stage of fat production known as ‘mitotic clonal expansion’. At this stage, preadipocytes are induced to proliferate, increasing their numbers. Later they accumulate a fat reservoir, growing in size to become mature adipocytes. As the levels of the short form of RUNX1T1 were much higher in the cells from the mice with Fto overexpressed, the researchers suggested that FTO must swing the balance so that more of the short form is produced. This in turn leads to a chain of events that result in more mitotic clonal expansion, more fat cells being produced and ultimately a mouse that gains weight more easily. Their work has therefore revealed a new function for FTO – increasing fat production. “Many early studies on FTO function focused on food intake,” Dr Dyan Sellayah, a senior author on the paper commented, “but this study is one of a growing number now that suggest that FTO may function in adipose tissue, independently of food intake.”