The ARRIVE guidelines aim to assure reproducibility and transparency in animal research. The International Mouse Phenotyping Consortium reports on how it meets them.
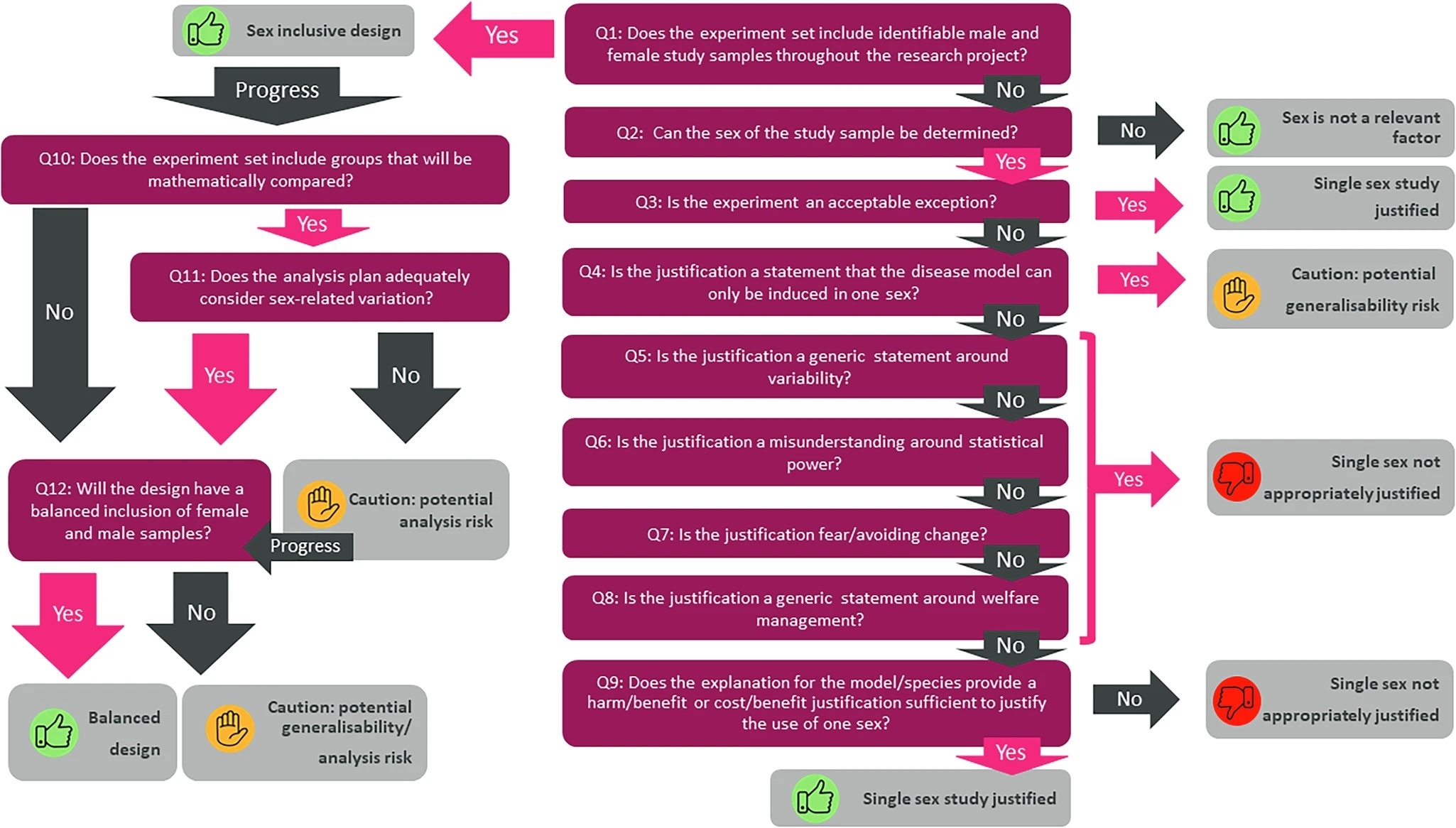
IMPC embraces ethical guidelines The ARRIVE guidelines, developed in 2010, aim to assure reproducibility and transparency in animal research. The IMPC reports on how it ensured it meets them. Reproducibility is fundamental to the success of scientific experimentation – the results of an experiment can only if be considered sound if they can be reproduced. Where animals are concerned, this is a particularly pressing issue. Solid science is based on sound ethics, and good experimental design leads to a reduction in animal numbers and improved animal welfare. The Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines, created by the National Centre for the Replacement, Refinement & Reduction of Animals in Research (NC3Rs), provide a checklist of 20 items that scientists use ensure their experiments are designed correctly and to report the details other scientists would need to obtain the same results. The ARRIVE guidelines were originally intended for those seeking to publish their findings in a peer-reviewed journal, but they can also be applied to large-scale databases. The International Mouse Phenotyping Consortium (IMPC), which is phenotyping over 20,000 mouse lines, follows these guidelines in the interest of maximising the transparency and reproducibility of their data. A detailed account of how is published in PLOS Biology. The consortium members began by distributing a survey to all of the IMPC centres, helping them assess how each centre carried out the experimental procedures. All details on how the data was obtained, such as the allele structure used and the genetic background of the mouse, were made available for download from the IMPC web portal. IMPC created a standardised language for key terms so that all centres could describe phenotypes in the same way. This allows cross-comparison of the data. They designed bespoke software packages for collecting IMPC data, and carrying out statistics to shed light on human disease. These measures ensured the IMPC’s work is in line with the ARRIVE guidelines. By following the ARRIVE guidelines, IMPC is doing all it can to remain a trustworthy, reliable source of phenotyping information for years to come. Karp NA, et al. (2015) Applying the ARRIVE guidelines to an In Vivo database. PLoS Biology. DOI: 10.1371/journal.pbio.1002151