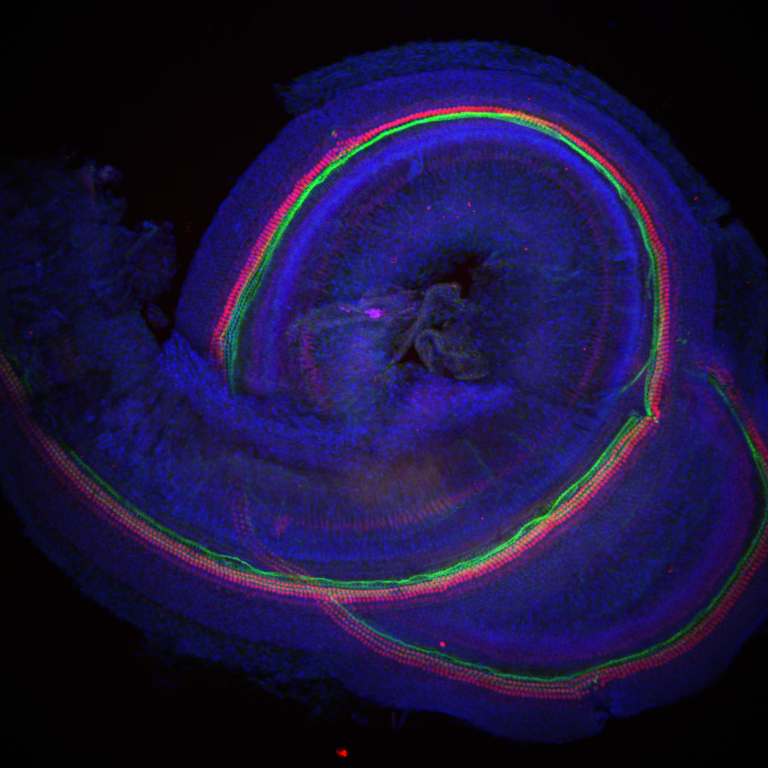
In research published in Nature, scientists at MRC Harwell and the University of Maryland (led by Dr Mike Bowl and Dr Ronna Hertzano, respectively) report the discovery of a key regulator which controls how an inner ear cell type critical for hearing, called outer hair cells, mature.
These new insights into hair cell maturation bring us a crucial step closer in the goal towards developing stem cell therapeutic approaches to treat hearing loss. In the UK, 1 in 6 people have a hearing deficit, with the elderly being most commonly affected. Age-related hearing loss is a worldwide problem and it is predicted by 2050 that more than 900 million people will suffer this condition. Experiencing hearing loss can have a profoundly negative impact on a person’s quality of life, both socially and economically. Our ability to hear depends on two types of specialized cells in the ear, known as inner and outer hair cells. These cells are responsible for turning sounds into electrical impulses and transmitting them to the brain. When these cells are damaged or lost, they are unable to regenerate and hearing becomes impaired, such as in the case of age-related hearing loss. Because of this, potential therapies to restore hearing have been focused on understanding the genetic roadmap that dictates how these hair cells develop and mature, as this could help initiate their replacement using stem cell therapy. Both inner and outer hair cells arise from a common type of precursor cell. Early in embryonic development, these cells begin to take different pathways in order to differentiate and mature into the two hair cell types. While the characteristics of these two cell types have been well defined, the genetic pathway to them becoming specialized is less clear. As part of a large screen undertaken at MRC Harwell, a mutation in the gene Ikzf2 was found to cause progressive hearing loss in mice. While this gene had previously been studied in other biological systems, a critical role in hearing was a novel finding. The Ikzf2 gene codes for the protein helios, which is a transcription factor and, as such, can regulate the expression of other genes. To explore how helios is required for hearing, studies were undertaken to see where and when the protein is present in the cochlea, the snail-shell like part of our inner ear that detects sound. This showed that helios is specifically found in immature outer hair cells, and is present at the point when they begin to mature into functional outer hair cells. This identified helios as a potential key component of outer hair cell specification. At the same time, a study was undertaken at the University of Maryland to assess gene expression across the inner ear, in order to learn more about the genetic landscape of the outer hair cells and what genes are required for their normal function. The results also supported a role for Ikzf2 in outer hair cell fate, finding that helios is likely required for the correct expression of many outer hair cell expressed genes. The team also collaborated with scientists from the University of Sheffield to take a closer look at how Ikzf2 may be involved in outer hair cell function. Outer hair cells act as cochlear amplifiers and one of their hallmark properties is the ability to contract and elongate. This process is known as electromotility and is critical for these cells to be able to amplify low-level sounds and function properly. Importantly, the outer hair cells with the Ikzf2 mutation showed significantly reduced electromotility. Furthermore, the researchers found that expression of two key outer hair cell-specific genes was reduced in mice with the Ikzf2 mutation compared to those without. Both of these genes code for proteins that are required for electromotility, reinforcing helios as an important factor regulating outer hair cell functional maturation. In the final part of the work, the team asked the question – what would happen if inner hair cells were made to express Ikzf2? Would this change their fate to become more like outer hair cells? They found that when inner hair cells were forced to produce helios, using a specially engineered virus, they showed reduced expression of inner hair cell-specific genes. In addition, they also displayed outer hair cell-like properties, such as increased expression of outer hair cell genes (including the two key outer hair cell-specific genes helios controls), and even electromotility. Crucially, this work provides evidence that we can manipulate cells and influence their fate in the ear. One of the authors and Director of the Institute Professor Steve Brown commented, “The development of therapies for age-related hearing loss represents one of the big challenges facing medicine and biomedical science. Understanding the genetic programmes that are responsible for the development and maturation of sound-transducing hair cells within the inner ear will be critical to exploring avenues for the regeneration of these cells that are lost in abundance during age-related hearing loss. The teams from the University of Maryland and MRC Harwell have given us the first insights into that programme. They have identified a master regulator, Ikzf2/helios, that controls the programme for maturation of outer hair cells. Now we have a target that we can potentially use to induce the production of outer hair cells within damaged inner ears, and we are one step closer to offering treatments for this disabling condition.” Dr Ralph Holme, Executive Director of Research at Action on Hearing Loss, the charity that helped fund the research said: “Despite 11 million people having hearing loss in the UK and the devastating impact it can have on the quality of life, treatments are still limited to hearing aids and cochlear implants. Therapeutics to restore hearing would completely transform lives for those who choose to use it, and we are excited to support this research which is an important step towards this huge goal.” This research represents a vital first step in identifying the regulatory factors that are required for hair cell specialization and could be used to direct stem cell development in therapy. Further work is now needed in order to identify additional factors that may be involved in regulating inner hair and outer hair cell development. Going forward, the helios transcription factor may eventually form part of a ‘cocktail’ of regulatory factors, which could be used in stem cell therapy to create new outer hair cells, replace damaged cells and ultimately help repair hearing loss. Image shows that Helios (stained in red) is specific to outer hair cells. A nuclear stain (blue) labels all cells, and an actin stain (green) binds to specialized structures found on both inner and outer hair cells, which are essential for sound detection. To read the research click here